Beryllium atoms have 4 electrons and the shell structure is 2.2. The ground state electron configuration of ground state gaseous neutral beryllium is He. 2s 2 and the term symbol is 1 S 0. Also know, what is a beryllium atom? Beryllium is a chemical element with the symbol Be and atomic number 4. As a free element it is a steel-gray, strong, lightweight and brittle alkaline earth metal. Also, what is the structure of a beryllium atom? The nucleus consists of 4 protons (red) and 5 neutrons (orange). The reactions of laser-ablated beryllium atoms with dinitrogen and carbon monoxide mixtures form the end-on bonded NNBeCO and side-on bonded (η 2 -N 2 )BeCO isomers in solid argon, which are predicted by quantum chemical calculations to be almost isoenergetic. Beryllium is used industrially in three forms: as a pure metal, as beryllium oxide, and most commonly, as an alloy with copper, aluminum, magnesium, or nickel. Beryllium oxide (called beryllia) is known for its high heat capacity and is an important component of certain sensitive electronic equipment. Hello, 1) The correct 'Lewis Dot Structure' for BeCl2 will have the 'least electronegative' element/Be in the center with two Cl 'single' ionic bonds (because this molecule involves a metal/cation and nonmetal/anion, thus there will be a 'transfer' of electrons to both Chlorines from Beryllium) with each Cl having 3 lone pairs (thus 6 electrons) around them.
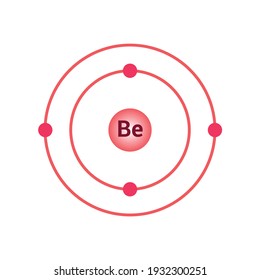
Beryllium has an atomic number 4 and is the first element of the 2nd group of the periodic table. It is classified as an alkaline earth metal. Beryllium is a comparatively rare element. It can be found in many gemstones. It has an atomic mass of 9.012 u and is represented by Be.
Beryllium is located in the S-block in the 2nd period. Thus its outermost electron is located in the s orbital. Beryllium has an electronic configuration of 1s2 2s2.
Beryllium is grey-white in color. It is relatively less dense and is, therefore, a lightweight metal. It has a hexagonal close-packed crystal structure. As all the electrons are paired it is diamagnetic (repels magnetic field lines).
Beryllium has a metallic radius of 111 pm.
All alkaline earth metals have low ionization enthalpies as they are electropositive. However, beryllium has the least radius among all the group two elements so it has comparatively high ionization enthalpy.
Beryllium does not impart any color to the flame during the flame test as its electrons are too strongly bound. Thus the electrons don’t get excited to higher levels even due to the flame, and thus no color can be seen. Beryllium has a high thermal conductivity.
Beryllium is both a common participant and a product in many nuclear reactions. It is present in the core of stars and it undergoes nuclear fusion to produce heavier elements. Beryllium was used in the discovery of the atomic structure and the discovery of the neutron by James Chadwick.
Chemical Properties of Beryllium
Beryllium is comparatively less reactive due to its completely filled outer s orbital. The beryllium atom is also small in size. However, it does participate in the following reactions.
With air

Powdered beryllium reacts with air to form BeO (beryllium oxide, amphoteric oxide) and Be3N2(beryllium intrude). Powdered beryllium burns in the air when ignited to form these compounds.
2Be + O2 -> 2BeO
3Be + N2 -> Be3N2
When beryllium reacts with oxygen or water, a very thin layer of beryllium oxide forms on the surface. This serves as a protective layer and the layers beneath this oxide do not react with the oxygen.
With halogens
All alkaline earth metals, including beryllium, react with halogen ( F, Cl, Br, I) at high temperatures thus resulting in the formation of metallic halides.
Be + X2 -> BeX2 where X=F, Cl, Br, I
With acids
Beryllium is located in group 2 and it’s an alkaline earth metal. Due to its electropositive nature, it is of the alkaline property. Thus beryllium reacts with acids liberating dihydrogen gas.
Be + 2HCl → BeCl2 + H2
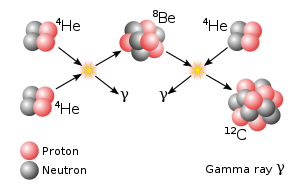
Reducing agent
Alkaline earth metals are electropositive in nature and thus can donate electrons acting as a good reducing agent.
But beryllium has a small radius and thus can’t donate electrons easily. So it has a less negative reduction potential (more negative reduction potential means more reducing power). However, due to the small size of Be2+ ion, it is stabilized easily by hydration energy. This gives beryllium it’s reducing nature as after donating electrons the ion is stabilized.
With hydrogen
Beryllium does not react with hydrogen on heating. But BeH2( beryllium hydride) can be produced in the following alternative method.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl2
Thus beryllium chloride is used. LiAlH4 is a very strong reducing agent and donates hydrogen easily.
Alternative methods to produce beryllium hydrides
Beryllium fluoride is produced from (NH4)2BeF4 by thermal decomposition.
Be(OH)2 + 2 (NH4)HF2 → (NH4)2BeF4 + 2 H2O
(NH4)2BeF4 → BeF2 + 2 NH3 + 2 HF
Beryllium chloride can be produced from beryllium oxide.
BeO+ C+ Cl2 →2 BeCl2 + CO
In liquid ammonia
All alkaline earth metals including beryllium dissolve in liquid ammonia. Solutions of ammoniated ions are formed which are deep blue-black in color.
With concentrated nitric acid
Beryllium resists the attack of concentrated nitric acid
Compounds of beryllium
The main oxidation state of beryllium is +2. Due to its small size, it forms predominantly covalent compounds.
Beryllium oxide forms on the reaction of oxygen with beryllium. It is amphoteric. It is quite stable to heat.
Beryllium hydroxide is amphoteric in nature.
Be(OH)2 + 2OH– → [Be(OH)4]2-
Beryllate ion is formed when beryllium hydroxide reacts with a base as shown above.
On reacting with acids
Be(OH)2 + 2HCl + 2H2O→[Be(OH)4]Cl2
Beryllium carbonate decomposes to give beryllium oxide and carbon dioxide. It is unstable at room temperature in the free atmosphere. It has to be stored in a CO2 rich atmosphere. Thus by Le Chatelier principle, the reaction shifts more to the left side.
BeCO3 = BeO + CO2
Beryllium halides are covalent and soluble in an organic solvent. Beryllium chloride has different structures in different phases. In the vapor state BeCl2 forms a chloro-bridged dimer which dissociates into the linear monomeric structure at high temperatures. In the solid-state Beryllium chloride ( BeCl2) has a chain structure.
Beryllium nitrate decomposes on heating to give beryllium oxide.
2Be(NO3)2 → 2BeO + 4NO2 + O2
Anomalous properties of Beryllium
Beryllium shows an anomalous behavior as
compared to the other members of Group 2.
Beryllium Atomic Group
Due to the small size of beryllium, it has higher ionization enthalpy. It also forms more covalent compounds.
The oxides and hydroxides of beryllium,
Beryllium Atomic Radius
are amphoteric in nature while the oxides and hydroxides of the other members in the group are basic.
The maximum coordination number that beryllium can exhibit is only 4 (like (BeF4)2-) as it has only 4 orbitals in its valence shell, unlike the other members of the group who can express a coordination number of six as they have d-orbitals.
Beryllium Atomic Weight
Many properties of beryllium are similar to aluminum and thus these two exhibits a diagonal relationship. They both have a similar ionic radius and charge/radius ratio. Both form protective oxide layers on their surface. Their chlorides are strong Lewis acids and are used as Friedel Craft catalysts.
Uses of Beryllium
- Beryllium was used to discover neutrons by James Chadwick. Beryllium was bombarded with alpha particles emitted from polonium which gave off a penetrating, electrically neutral radiation. It was proved that these emissions consisted of a particle with a mass similar to that of a proton. This was named as a neutron.
- Beryllium is popularly used as an alloying agent. Copper-beryllium alloys are used to make high strength springs, welding electrodes, electrical contacts in phones, etc.
- Metallic beryllium is quite transparent to X-rays, so thin Be-foil is used for making windows in X-ray tubes.
- Beryllium is a lightweight metal and also has a very high melting point. Thus it finds use in aircraft, satellites, space crafts, etc.
- Beryllium is used in nuclear reactors as a moderator and neutron reflectors. It has high thermal conductivity and is transparent to most forms of radiation.
- Beryllium is non-sparking, temperature-resistant metal and is thus used to make tools and instruments in the oil and automotive industry.
- Other uses include windshield frame, support beams, brakes, etc. where lightweight but stiff material is required.
