Click a column header, such as Name, to sort the table by that item.
SEENotes at the bottom of the Table.
- If you have an element name or symbol, use a periodic table to find the atomic.
- The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that.
What does atomic-number mean? The number of protons in an atomic nucleus. Or proton number (Z) The number of protons in the nucleus of an atom. The number of atoms or molecules (n) in a mass (m) of a pure material having atomic or molecular weight (M) is easily computed from the following equation using Avogadro's number (NA = 6.022×10 23 atoms or molecules per gram-mole): M mN n A (1) In some situations, the atomic number density (N), which is the concentration of atoms or molecules per.
| No. | Atomic weight | Name | Sym. | M.P. (°C) | B.P. (°C) | Density* (g/cm3) | Earth crust (%)* | Discovery (Year) | Group* | Electron configuration | Ionization energy (eV) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.008 | Hydrogen | H | -259 | -253 | 0.09 | 0.14 | 1776 | 1 | 1s1 | 13.60 | |
| 2 | 4.003 | Helium | He | -272 | -269 | 0.18 | 1895 | 18 | 1s2 | 24.59 | ||
| 3 | 6.941 | Lithium | Li | 180 | 1,347 | 0.53 | 1817 | 1 | [He] 2s1 | 5.39 | ||
| 4 | 9.012 | Beryllium | Be | 1,278 | 2,970 | 1.85 | 1797 | 2 | [He] 2s2 | 9.32 | ||
| 5 | 10.811 | Boron | B | 2,300 | 2,550 | 2.34 | 1808 | 13 | [He] 2s2 2p1 | 8.30 | ||
| 6 | 12.011 | Carbon | C | 3,500 | 4,827 | 2.26 | 0.09 | ancient | 14 | [He] 2s2 2p2 | 11.26 | |
| 7 | 14.007 | Nitrogen | N | -210 | -196 | 1.25 | 1772 | 15 | [He] 2s2 2p3 | 14.53 | ||
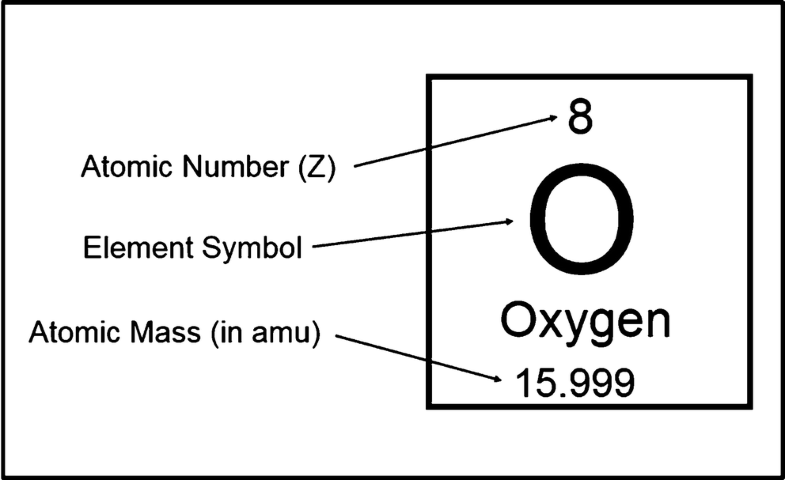
| 8 | 15.999 | Oxygen | O | -218 | -183 | 1.43 | 46.71 | 1774 | 16 | [He] 2s2 2p4 | 13.62 | |
| 9 | 18.998 | Fluorine | F | -220 | -188 | 1.70 | 0.03 | 1886 | 17 | [He] 2s2 2p5 | 17.42 | |
| 10 | 20.180 | Neon | Ne | -249 | -246 | 0.90 | 1898 | 18 | [He] 2s2 2p6 | 21.56 | ||
| 11 | 22.990 | Sodium | Na | 98 | 883 | 0.97 | 2.75 | 1807 | 1 | [Ne] 3s1 | 5.14 | |
| 12 | 24.305 | Magnesium | Mg | 639 | 1,090 | 1.74 | 2.08 | 1755 | 2 | [Ne] 3s2 | 7.65 | |
| 13 | 26.982 | Aluminum | Al | 660 | 2,467 | 2.70 | 8.07 | 1825 | 13 | [Ne] 3s2 3p1 | 5.99 | |
| 14 | 28.086 | Silicon | Si | 1,410 | 2,355 | 2.33 | 27.69 | 1824 | 14 | [Ne] 3s2 3p2 | 8.15 | |
| 15 | 30.974 | Phosphorus | P | 44 | 280 | 1.82 | 0.13 | 1669 | 15 | [Ne] 3s2 3p3 | 10.49 | |
| 16 | 32.065 | Sulfur | S | 113 | 445 | 2.07 | 0.05 | ancient | 16 | [Ne] 3s2 3p4 | 10.36 | |
| 17 | 35.453 | Chlorine | Cl | -101 | -35 | 3.21 | 0.05 | 1774 | 17 | [Ne] 3s2 3p5 | 12.97 | |
| 18 | 39.948 | Argon | Ar | -189 | -186 | 1.78 | 1894 | 18 | [Ne] 3s2 3p6 | 15.76 | ||
| 19 | 39.098 | Potassium | K | 64 | 774 | 0.86 | 2.58 | 1807 | 1 | [Ar] 4s1 | 4.34 | |
| 20 | 40.078 | Calcium | Ca | 839 | 1,484 | 1.55 | 3.65 | 1808 | 2 | [Ar] 4s2 | 6.11 | |
| 21 | 44.956 | Scandium | Sc | 1,539 | 2,832 | 2.99 | 1879 | 3 | [Ar] 3d1 4s2 | 6.56 | ||
| 22 | 47.867 | Titanium | Ti | 1,660 | 3,287 | 4.54 | 0.62 | 1791 | 4 | [Ar] 3d2 4s2 | 6.83 | |
| 23 | 50.942 | Vanadium | V | 1,890 | 3,380 | 6.11 | 1830 | 5 | [Ar] 3d3 4s2 | 6.75 | ||
| 24 | 51.996 | Chromium | Cr | 1,857 | 2,672 | 7.19 | 0.04 | 1797 | 6 | [Ar] 3d5 4s1 | 6.77 | |
| 25 | 54.938 | Manganese | Mn | 1,245 | 1,962 | 7.43 | 0.09 | 1774 | 7 | [Ar] 3d5 4s2 | 7.43 | |
| 26 | 55.845 | Iron | Fe | 1,535 | 2,750 | 7.87 | 5.05 | ancient | 8 | [Ar] 3d6 4s2 | 7.90 | |
| 27 | 58.933 | Cobalt | Co | 1,495 | 2,870 | 8.90 | 1735 | 9 | [Ar] 3d7 4s2 | 7.88 | ||
| 28 | 58.693 | Nickel | Ni | 1,453 | 2,732 | 8.90 | 0.02 | 1751 | 10 | [Ar] 3d8 4s2 | 7.64 | |
| 29 | 63.546 | Copper | Cu | 1,083 | 2,567 | 8.96 | ancient | 11 | [Ar] 3d10 4s1 | 7.73 | ||
| 30 | 65.390 | Zinc | Zn | 420 | 907 | 7.13 | ancient | 12 | [Ar] 3d10 4s2 | 9.39 | ||
| 31 | 69.723 | Gallium | Ga | 30 | 2,403 | 5.91 | 1875 | 13 | [Ar] 3d10 4s2 4p1 | 6.00 | ||
| 32 | 72.640 | Germanium | Ge | 937 | 2,830 | 5.32 | 1886 | 14 | [Ar] 3d10 4s2 4p2 | 7.90 | ||
| 33 | 74.922 | Arsenic | As | 81 | 613 | 5.72 | ancient | 15 | [Ar] 3d10 4s2 4p3 | 9.79 | ||
| 34 | 78.960 | Selenium | Se | 217 | 685 | 4.79 | 1817 | 16 | [Ar] 3d10 4s2 4p4 | 9.75 | ||
| 35 | 79.904 | Bromine | Br | -7 | 59 | 3.12 | 1826 | 17 | [Ar] 3d10 4s2 4p5 | 11.81 | ||
| 36 | 83.800 | Krypton | Kr | -157 | -153 | 3.75 | 1898 | 18 | [Ar] 3d10 4s2 4p6 | 14.00 | ||
| 37 | 85.468 | Rubidium | Rb | 39 | 688 | 1.63 | 1861 | 1 | [Kr] 5s1 | 4.18 | ||
| 38 | 87.620 | Strontium | Sr | 769 | 1,384 | 2.54 | 1790 | 2 | [Kr] 5s2 | 5.69 | ||
| 39 | 88.906 | Yttrium | Y | 1,523 | 3,337 | 4.47 | 1794 | 3 | [Kr] 4d1 5s2 | 6.22 | ||
| 40 | 91.224 | Zirconium | Zr | 1,852 | 4,377 | 6.51 | 0.03 | 1789 | 4 | [Kr] 4d2 5s2 | 6.63 | |
| 41 | 92.906 | Niobium | Nb | 2,468 | 4,927 | 8.57 | 1801 | 5 | [Kr] 4d4 5s1 | 6.76 | ||
| 42 | 95.940 | Molybdenum | Mo | 2,617 | 4,612 | 10.22 | 1781 | 6 | [Kr] 4d5 5s1 | 7.09 | ||
| 43 | * | 98.000 | Technetium | Tc | 2,200 | 4,877 | 11.50 | 1937 | 7 | [Kr] 4d5 5s2 | 7.28 | |
| 44 | 101.070 | Ruthenium | Ru | 2,250 | 3,900 | 12.37 | 1844 | 8 | [Kr] 4d7 5s1 | 7.36 | ||
| 45 | 102.906 | Rhodium | Rh | 1,966 | 3,727 | 12.41 | 1803 | 9 | [Kr] 4d8 5s1 | 7.46 | ||
| 46 | 106.420 | Palladium | Pd | 1,552 | 2,927 | 12.02 | 1803 | 10 | [Kr] 4d10 | 8.34 | ||
| 47 | 107.868 | Silver | Ag | 962 | 2,212 | 10.50 | ancient | 11 | [Kr] 4d10 5s1 | 7.58 | ||
| 48 | 112.411 | Cadmium | Cd | 321 | 765 | 8.65 | 1817 | 12 | [Kr] 4d10 5s2 | 8.99 | ||
| 49 | 114.818 | Indium | In | 157 | 2,000 | 7.31 | 1863 | 13 | [Kr] 4d10 5s2 5p1 | 5.79 | ||
| 50 | 118.710 | Tin | Sn | 232 | 2,270 | 7.31 | ancient | 14 | [Kr] 4d10 5s2 5p2 | 7.34 | ||
| 51 | 121.760 | Antimony | Sb | 630 | 1,750 | 6.68 | ancient | 15 | [Kr] 4d10 5s2 5p3 | 8.61 | ||
| 52 | 127.600 | Tellurium | Te | 449 | 990 | 6.24 | 1783 | 16 | [Kr] 4d10 5s2 5p4 | 9.01 | ||
| 53 | 126.905 | Iodine | I | 114 | 184 | 4.93 | 1811 | 17 | [Kr] 4d10 5s2 5p5 | 10.45 | ||
| 54 | 131.293 | Xenon | Xe | -112 | -108 | 5.90 | 1898 | 18 | [Kr] 4d10 5s2 5p6 | 12.13 | ||
| 55 | 132.906 | Cesium | Cs | 29 | 678 | 1.87 | 1860 | 1 | [Xe] 6s1 | 3.89 | ||
| 56 | 137.327 | Barium | Ba | 725 | 1,140 | 3.59 | 0.05 | 1808 | 2 | [Xe] 6s2 | 5.21 | |
| 57 | 138.906 | Lanthanum | La | 920 | 3,469 | 6.15 | 1839 | 3 | [Xe] 5d1 6s2 | 5.58 | ||
| 58 | 140.116 | Cerium | Ce | 795 | 3,257 | 6.77 | 1803 | 101 | [Xe] 4f1 5d1 6s2 | 5.54 | ||
| 59 | 140.908 | Praseodymium | Pr | 935 | 3,127 | 6.77 | 1885 | 101 | [Xe] 4f3 6s2 | 5.47 | ||
| 60 | 144.240 | Neodymium | Nd | 1,010 | 3,127 | 7.01 | 1885 | 101 | [Xe] 4f4 6s2 | 5.53 | ||
| 61 | * | 145.000 | Promethium | Pm | 1,100 | 3,000 | 7.30 | 1945 | 101 | [Xe] 4f5 6s2 | 5.58 | |
| 62 | 150.360 | Samarium | Sm | 1,072 | 1,900 | 7.52 | 1879 | 101 | [Xe] 4f6 6s2 | 5.64 | ||
| 63 | 151.964 | Europium | Eu | 822 | 1,597 | 5.24 | 1901 | 101 | [Xe] 4f7 6s2 | 5.67 | ||
| 64 | 157.250 | Gadolinium | Gd | 1,311 | 3,233 | 7.90 | 1880 | 101 | [Xe] 4f7 5d1 6s2 | 6.15 | ||
| 65 | 158.925 | Terbium | Tb | 1,360 | 3,041 | 8.23 | 1843 | 101 | [Xe] 4f9 6s2 | 5.86 | ||
| 66 | 162.500 | Dysprosium | Dy | 1,412 | 2,562 | 8.55 | 1886 | 101 | [Xe] 4f10 6s2 | 5.94 | ||
| 67 | 164.930 | Holmium | Ho | 1,470 | 2,720 | 8.80 | 1867 | 101 | [Xe] 4f11 6s2 | 6.02 | ||
| 68 | 167.259 | Erbium | Er | 1,522 | 2,510 | 9.07 | 1842 | 101 | [Xe] 4f12 6s2 | 6.11 | ||
| 69 | 168.934 | Thulium | Tm | 1,545 | 1,727 | 9.32 | 1879 | 101 | [Xe] 4f13 6s2 | 6.18 | ||
| 70 | 173.040 | Ytterbium | Yb | 824 | 1,466 | 6.90 | 1878 | 101 | [Xe] 4f14 6s2 | 6.25 | ||
| 71 | 174.967 | Lutetium | Lu | 1,656 | 3,315 | 9.84 | 1907 | 101 | [Xe] 4f14 5d1 6s2 | 5.43 | ||
| 72 | 178.490 | Hafnium | Hf | 2,150 | 5,400 | 13.31 | 1923 | 4 | [Xe] 4f14 5d2 6s2 | 6.83 | ||
| 73 | 180.948 | Tantalum | Ta | 2,996 | 5,425 | 16.65 | 1802 | 5 | [Xe] 4f14 5d3 6s2 | 7.55 | ||
| 74 | 183.840 | Tungsten | W | 3,410 | 5,660 | 19.35 | 1783 | 6 | [Xe] 4f14 5d4 6s2 | 7.86 | ||
| 75 | 186.207 | Rhenium | Re | 3,180 | 5,627 | 21.04 | 1925 | 7 | [Xe] 4f14 5d5 6s2 | 7.83 | ||
| 76 | 190.230 | Osmium | Os | 3,045 | 5,027 | 22.60 | 1803 | 8 | [Xe] 4f14 5d6 6s2 | 8.44 | ||
| 77 | 192.217 | Iridium | Ir | 2,410 | 4,527 | 22.40 | 1803 | 9 | [Xe] 4f14 5d7 6s2 | 8.97 | ||
| 78 | 195.078 | Platinum | Pt | 1,772 | 3,827 | 21.45 | 1735 | 10 | [Xe] 4f14 5d9 6s1 | 8.96 | ||
| 79 | 196.967 | Gold | Au | 1,064 | 2,807 | 19.32 | ancient | 11 | [Xe] 4f14 5d10 6s1 | 9.23 | ||
| 80 | 200.590 | Mercury | Hg | -39 | 357 | 13.55 | ancient | 12 | [Xe] 4f14 5d10 6s2 | 10.44 | ||
| 81 | 204.383 | Thallium | Tl | 303 | 1,457 | 11.85 | 1861 | 13 | [Xe] 4f14 5d10 6s2 6p1 | 6.11 | ||
| 82 | 207.200 | Lead | Pb | 327 | 1,740 | 11.35 | ancient | 14 | [Xe] 4f14 5d10 6s2 6p2 | 7.42 | ||
| 83 | 208.980 | Bismuth | Bi | 271 | 1,560 | 9.75 | ancient | 15 | [Xe] 4f14 5d10 6s2 6p3 | 7.29 | ||
| 84 | * | 209.000 | Polonium | Po | 254 | 962 | 9.30 | 1898 | 16 | [Xe] 4f14 5d10 6s2 6p4 | 8.42 | |
| 85 | * | 210.000 | Astatine | At | 302 | 337 | 0.00 | 1940 | 17 | [Xe] 4f14 5d10 6s2 6p5 | 9.30 | |
| 86 | * | 222.000 | Radon | Rn | -71 | -62 | 9.73 | 1900 | 18 | [Xe] 4f14 5d10 6s2 6p6 | 10.75 | |
| 87 | * | 223.000 | Francium | Fr | 27 | 677 | 0.00 | 1939 | 1 | [Rn] 7s1 | 4.07 | |
| 88 | * | 226.000 | Radium | Ra | 700 | 1,737 | 5.50 | 1898 | 2 | [Rn] 7s2 | 5.28 | |
| 89 | * | 227.000 | Actinium | Ac | 1,050 | 3,200 | 10.07 | 1899 | 3 | [Rn] 6d1 7s2 | 5.17 | |
| 90 | 232.038 | Thorium | Th | 1,750 | 4,790 | 11.72 | 1829 | 102 | [Rn] 6d2 7s2 | 6.31 | ||
| 91 | 231.036 | Protactinium | Pa | 1,568 | 0 | 15.40 | 1913 | 102 | [Rn] 5f2 6d1 7s2 | 5.89 | ||
| 92 | 238.029 | Uranium | U | 1,132 | 3,818 | 18.95 | 1789 | 102 | [Rn] 5f3 6d1 7s2 | 6.19 | ||
| 93 | * | 237.000 | Neptunium | Np | 640 | 3,902 | 20.20 | 1940 | 102 | [Rn] 5f4 6d1 7s2 | 6.27 | |
| 94 | * | 244.000 | Plutonium | Pu | 640 | 3,235 | 19.84 | 1940 | 102 | [Rn] 5f6 7s2 | 6.03 | |
| 95 | * | 243.000 | Americium | Am | 994 | 2,607 | 13.67 | 1944 | 102 | [Rn] 5f7 7s2 | 5.97 | |
| 96 | * | 247.000 | Curium | Cm | 1,340 | 0 | 13.50 | 1944 | 102 | 5.99 | ||
| 97 | * | 247.000 | Berkelium | Bk | 986 | 0 | 14.78 | 1949 | 102 | 6.20 | ||
| 98 | * | 251.000 | Californium | Cf | 900 | 0 | 15.10 | 1950 | 102 | 6.28 | ||
| 99 | * | 252.000 | Einsteinium | Es | 860 | 0 | 0.00 | 1952 | 102 | 6.42 | ||
| 100 | * | 257.000 | Fermium | Fm | 1,527 | 0 | 0.00 | 1952 | 102 | 6.50 | ||
| 101 | * | 258.000 | Mendelevium | Md | 0 | 0 | 0.00 | 1955 | 102 | 6.58 | ||
| 102 | * | 259.000 | Nobelium | No | 827 | 0 | 0.00 | 1958 | 102 | 6.65 | ||
| 103 | * | 262.000 | Lawrencium | Lr | 1,627 | 0 | 0.00 | 1961 | 102 | 4.90 | ||
| 104 | * | 261.000 | Rutherfordium | Rf | 0 | 0 | 0.00 | 1964 | 4 | 0.00 | ||
| 105 | * | 262.000 | Dubnium | Db | 0 | 0 | 0.00 | 1967 | 5 | 0.00 | ||
| 106 | * | 266.000 | Seaborgium | Sg | 0 | 0 | 0.00 | 1974 | 6 | 0.00 | ||
| 107 | * | 264.000 | Bohrium | Bh | 0 | 0 | 0.00 | 1981 | 7 | 0.00 | ||
| 108 | * | 277.000 | Hassium | Hs | 0 | 0 | 0.00 | 1984 | 8 | 0.00 | ||
| 109 | * | 268.000 | Meitnerium | Mt | 0 | 0 | 0.00 | 1982 | 9 | 0.00 | ||
| No. | Atomic weight | Name | Sym. | M.P. (°C) | B.P. (°C) | Density* (g/cm3) | Earth crust (%)* | Discovery (Year) | Group* | Electron configuration | Ionization energy (eV) |
Notes:
• Density of elements with boiling points below 0°C is given in g/l. In a sorted list, these elements are shown before other elements that have boiling points >0°C.
• Earth crust composition average values are from a report by F. W. Clarke and H. S. Washington, 1924. Elemental composition of crustal rocks differ between different localities (see article).
• Group: There are only 18 groups in the periodic table that constitute the columns of the table. Lanthanoids and Actinoids are numbered as 101 and 102 to separate them in sorting by group.
• The elements marked with an asterisk (in the 2nd column) have no stable nuclides. For these elements the weight value shown represents the mass number of the longest-lived isotope of the element.

Abbreviations and Definitions:
The Atomic Number Tells You The Number Of
No. - Atomic Number; M.P. - melting point; B.P. - boiling point
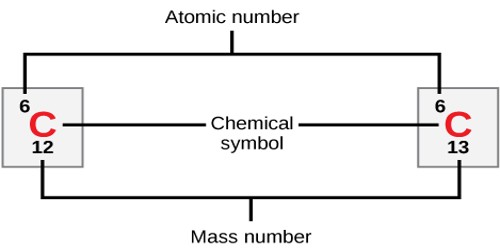
Atomic number: The number of protons in an atom. Each element is uniquely defined by its atomic number.
Atomic mass: The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Atomic mass is measured in Atomic Mass Units (amu) which are scaled relative to carbon, 12C, that is taken as a standard element with an atomic mass of 12. This isotope of carbon has 6 protons and 6 neutrons. Thus, each proton and neutron has a mass of about 1 amu.
Isotope: Atoms of the same element with the same atomic number, but different number of neutrons. Isotope of an element is defined by the sum of the number of protons and neutrons in its nucleus. Elements have more than one isotope with varying numbers of neutrons. For example, there are two common isotopes of carbon, 12C and 13C which have 6 and 7 neutrons respectively. The abundances of different isotopes of elements vary in nature depending on the source of materials. For relative abundances of isotopes in nature see reference on Atomic Weights and Isotopic Compositions.
Atomic weight: Atomic weight values represent weighted average of the masses of all naturally occurring isotopes of an element. The values shown here are based on the IUPAC Commission determinations (Pure Appl. Chem. 73:667-683, 2001). The elements marked with an asterisk have no stable nuclides. For these elements the weight value shown represents the mass number of the longest-lived isotope of the element.
The Atomic Number Of An Element Is The Number Of
Electron configuration: See next page for explanation of electron configuration of atoms.
Ionization energy (IE): The energy required to remove the outermost electron from an atom or a positive ion in its ground level. The table lists only the first IE in eV units. To convert to kJ/mol multiply by 96.4869. Reference: NIST Reference Table on Ground states and ionization energies for the neutral atoms. IE decreases going down a column of the periodic table, and increases from left to right in a row. Thus, alkali metals have the lowest IE in a period and Rare gases have the highest.
Other resources related to the Periodic Table
- Chemical Evolution of the Universe
